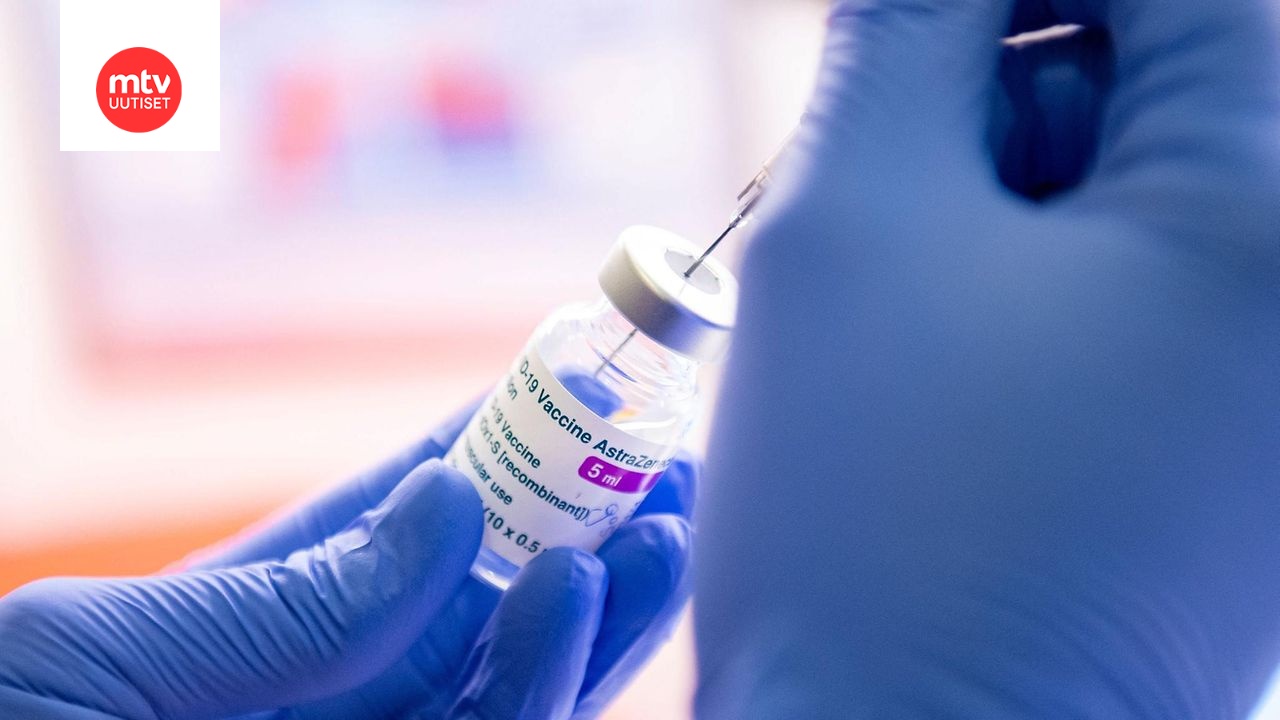
Ihmisten kyvyn hahmottaa numeroiden suuruutta sanotaan vaikeutuvan huomattavasti, kun puhutaan luvuista jotka ovat yli kaksin- kolminkertaisia omiin kuukausituloihin nähden. Tämä näyttäisi olevan totta myös tässä ketjussa, sillä niin älytöntä AZ sekä J&J rokotteiden mollaamista ei voi selittää kuin todennäköisyyden ymmärtämättömyydellä tai trollaamisella. Kaikissa lääkkeissä on riskinsä, ja veikkaan myös kritisoijien hyväksyneen omassa historiassaan huomattavasti todennäköisempiä vakavia riskejä niiden kanssa. Otetaan muutama esimerkki vakavista haittavaikutuksista ja niiden todennäköisyyksistä:
Para tabs (parasetamoli)
- Anemia (alle yhdelle tuhannesta, yli yhdelle kymmenestä tuhannesta)
- henkeä uhkaava yliherkkyysreaktio(anafylaktinen sokki); toksinen epidermaalinen nekrolyysi (alle yhdelle tuhannesta)
Doximycin (yleinen antibiootti)
- Sydänpussitulehdus (yhdelle tuhannesta)
- Maksatulehdus (yhdelle tuhannesta)
Panacod (kipulääke)
- Haimatulehdus (yhdelle kymmenestä tuhannesta)
Burana (kipulääke)
- Kuulon alenema (yhdelle kymmenestä tuhannesta)
Listaa voisi jatkaa loputtomiin. Mahdollisuus saada rokotteesta verenhyytymä on jotain 1/100 000 ja 1/1 000 000 välissä, eli käytännössä niin pieni että yhtä todennäköisesti saan osuman salamaniskusta. (0,000012% eli noin 1/1 000 000 lähde:
Kumpi todennäköisempää: Lottovoitto vai salamanisku?)
Jos on valmis ottamaan huomattavasti suuremman riskin syömällä Buranaa, en ymmärrä valitusta rokotteen suurista haittavaikutuksista. Ja Buranan kanssa sen riskin ottaa joka kerta sitä käyttäessä, rokotusta ei tarvitse näillä näkymin kuin kerran tai kaksi. En kiellä etteikö haittoja ole, ja tietyillä lähtökohdilla niiden todennäköisyys voi olla suurempi. Vielä yhteistä nimittäjää tai nimittäjiä ei ole löydetty, mutta eiköhän sellaisiakin vielä tule vastaan kun tutkimukset etenevät. Mitään niin huolestuttavaa ei ole kuitenkaan vielä tullut ilmi, että näiden rokotteiden käyttö pitäisi lopettaa täysin.

www.is.fi

www.is.fi